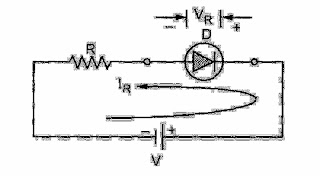
To understand the various switching times, consider simple diode circuit and an input waveform as shown in the Fig.
Simple diode circuit
The following events will take place due to the nature of the applied voltage
Event 1: Till time t the forward voltage applied is VF and diode is forward biased. The value of R is large enough such that drop across forward biased diode is very small compared to drop across R. The forward current is then
neglecting
forward resistance of diode.
Event 2: At time t the applied voltage is suddenly reversed and reverse voltage of -
VR is applied to the circuit. Ideally diode also must become OFF from ON state instantly. But this does not happen instantly.
The number of minority carriers take time to reduce from p no to zero at the junction Due to this, at t1 current just reverses and remains at that reversed value IR till the minority carrier concentration reduces to zero. This current is given by -IR = -VR / R. This continues to flow till time t
Event3: From t onwards, the diode voltage starts to reverse and the diode current
starts decreasing as shown in the Fig. At t = t; the diode state completely gets reversed and attains steady state in reverse biased condition.
The total time required by the diode which is the sum of storage time and transition time, to recover completely from the change of state is called reverse recovery time
of the diode and denoted as trr. This is an important consideration in high speed switching applications.
The reverse recovery time depends on the RC time constant where C is a transition capacitance of a diode. Thus the transition capacitance plays an important role in the switching circuits using diodes.The total switching time trr puts the limit on the maximum operating frequency of the diode. Hence trr is an important datasheet specification. To minimize the effect of the reverse current, the time period of the operating frequency must be at least ten times trr.
where fmax is the maximum operating frequency.