A solid is determined as a conductor, insulator or semiconductor by the energy band structure.
Conductors
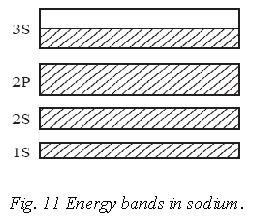
Thus if there are N atoms in a solid piece of sodium, its 3s valence band will contain N energy levels can hold 2N electrons. Thus the 3s band in sodium is only half filled by electrons and the Fermi energy EF lies in the middle of the band. When a potential difference is applied across a piece of solid sodium, 3s electrons can pick up additional energy while remaining in their original band. The additional energy is in the form of KE, and the drift of the electrons constitute an electric current. Sodium is therefore a good conductor.
Insulators
Semiconductors
Silicon has a crystal structure like diamond; a gap separates the top of its filled valence band from an empty conduction band above it. The band gap in silicon however is only about 1eV wide. At low temperature silicon is little better than diamond as a conductor, but at room temperature a small number of its valence electrons have enough thermal energy to jump the forbidden band and enter the conduction band.
These electrons, though few, are still enough to allow a small amount of current to flow when an electric field is applied. Thus silicon has a resistivity intermediate between those of conductors and those of insulators and is called as semiconductors. The energy band diagram of semiconductor is given in Fig. 13.


No comments:
Post a Comment